Welcome to Medealis
Our special expertise in polymers and plastic injection molding has proven particularly valuable, especially in the development of retention inserts. This is a critical component for the function of any removable prosthesis.
Our focus is on the highest quality and modern production processes allowing us to offer our customers worldwide, high-tech implantology products “Made in Germany”, at an attractive price.
We look forward to introducing you to our products and solutions and helping to provide the best possible restorations for patients. Please do not hesitate to contact us if you need further information or have any questions.
Klaus Krueger
Managing Director
Quality „Made in Germany”
Our strict quality standards also include the conditions under which our products are manufactured. This is another reason why we manufacture in Germany, where fair working conditions are ensured.

Our Products
Patented Biocompatible Coating
Our tremendously hard coating of Zirconium Carbon Nitride (ZrCN) provides many advantages over alternative abutment coatings.
- Increased resistance to wear and abrasion
- Ceramic PVD (Physical Vapor Deposition) coating that minimizes the accumulation of plaque
- Rose gold tone blends well with the gingiva and provides a premium appearance
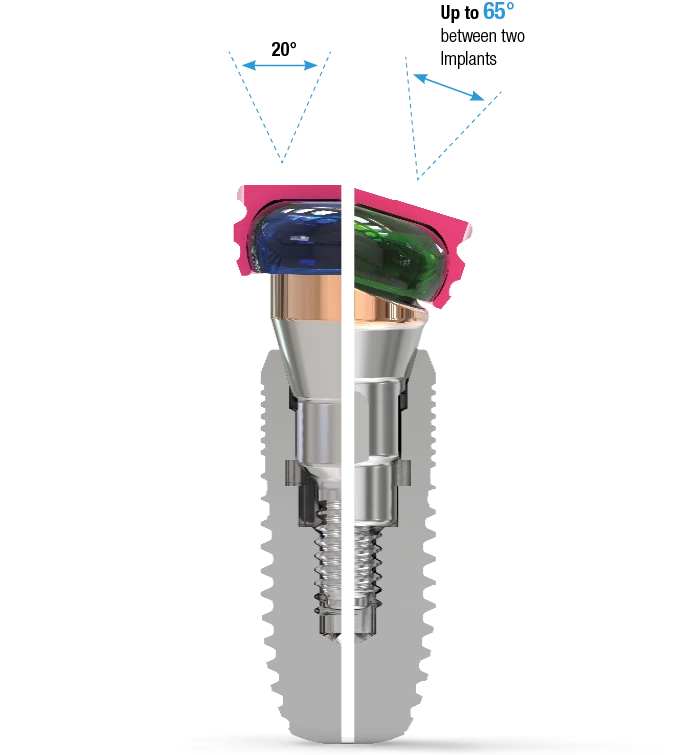
The 18° Angled Abutment
This angulation is the solution for many clinical implant situations:
- Compensates for off-axis implant placement
- Allows for the restoration of implants with up to 65° of divergence between them
- Available in two connection variants
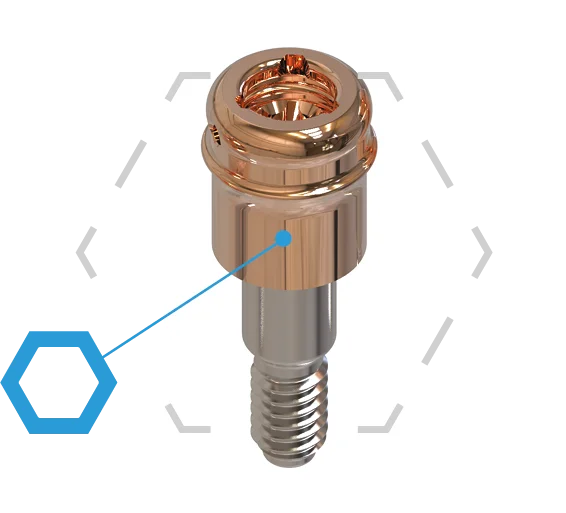
Compatible Connections
Our abutments are compatible with most commonly used screwdrivers
- No additional tooling required
- Compatible Tri-Lobe drive mechanism
- Features a 1,25mm hex drive mechanism in the center of the abutment
- Simple seating
High Performance Retention Inserts
More comfort for patients: our High Performance Retention Inserts made of polyamide are extremely durable and have an excellent dynamic load capacity.
- High resistance to chemicals, fats and alcoholic disinfectants
- Low tendency to absorb moisture
- Suitable for steam sterilization at 134°C
- Ensure stable denture retention over time

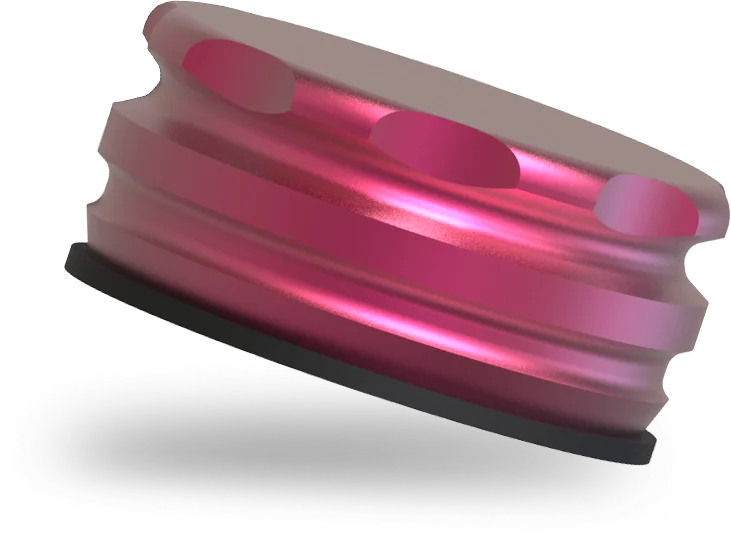
Improved Denture Caps
Our products are compatible
with implant systems from the following manufacturers:
Bego
botticelli
bredent medical
Camlog
C-TECH IMPLANT
Dentsply Sirona
LASAK
LOGON
MegaGen
OSSTEM
Southern Implants
Straumann®
ZimVie
System tools and accessories
Bar Components
Some reviews from our Key Opinion Leaders
Docklocs® 18 ̊ angled abutments provide me with a solution for off-axis implant placement. The improved polyamide inserts, and housing deliver stable retention of the denture. Placement of the attachments is easy, and the Docklocs® system is compatible.”
Docklocs® offers me compatibility, reliable denture retention and a superior abutment coating. With the 18 ̊ angled abutments accommodating greater implant angulation, a broader number of patients can be successfully treated.”
The combination of the esthetic Docklocs® abutments and addition of the 18 ̊ angled abutments has increased the number of cases that I can confidently restore. All components are easy to use, compatible and our patients are happy!”

Prof. Dr. Jörg Neugebauer
Oral Surgeon, Professorship Digitalization in Dentistry, Steinbeis University, Berlin
President, Academy of Osseointegration, Chicago, USA

Prof. Dr. Hans-Florian Zeilhofer
Chairman of Board of Directors and co-founder of the Botticelli Implant System, Former head of the Clinic for Oral and Maxillofacial Surgery at University Hospital Basel and Aarau

Gerhard Neuendorff
Master Dental Technician, Germany

Prof. Dr. Jörg Neugebauer
Oral Surgeon, Professorship Digitalization in Dentistry, Steinbeis University, Berlin
President, Academy of Osseointegration, Chicago, USA

Prof. Dr. Hans-Florian Zeilhofer
Chairman of Board of Directors and co-founder of the Botticelli Implant System, Former head of the Clinic for Oral and Maxillofacial Surgery at University Hospital Basel and Aarau

Gerhard Neuendorff
Master Dental Technician, Germany
Instructions for Use
| DE | Gebrauchsanweisung und relevante Produktinformationen |
| EN | Instructions for Use and relevant product information |
| FR | Mode d‘emploi et informations pertinentes sur le produit |
| IT | Istruzioni per l’uso e informazioni rilevanti sul prodotto |
| PT | Instruções de uso e informações relevantes sobre o produto |
| ES | Instrucciones de uso e información pertinente sobre el producto |
Certificates
Education
Product Videos
Please don’t hesitate to contact us if you have a request.
info@medealis.de